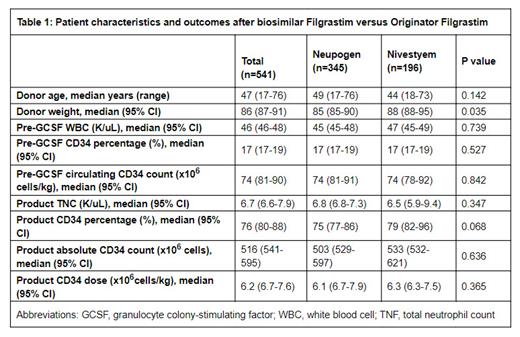
Background: Allogeneic hematopoietic stem cell transplantation (HSCT) is an optimal and potentially curative therapy for patients with hematologic malignancies. Granulocyte colony-stimulating factor (G-CSF) is used for the mobilization of peripheral blood stem cells (PBSCs) in allogeneic donors, and an optimal CD34 cell dose plays a critical role in successful transplantation. Nivestym, a biosimilar G-CSF to the originator Filgrastim (Neupogen), is now used by multiple institutions as it has become more available; however, there is a lack of data in this regard among healthy donors for allogeneic HSCT mobilization. In this study, we aim to compare the efficacy of Nivestym compared to Neupogen for allogeneic donor PBSC mobilization. Methods: For this retrospective single-center study, we included all (n=541) adult allogeneic HSCT donors at the University of Kansas Medical Center receiving Nivestym (January 2013-July 2020) or Neupogen (July 2020-June 2023) for donor PBSC mobilization. We conducted a bivariate analysis utilizing the ANOVA test. Statistical analyses were performed using SPSS version 28. Statistical significance was determined at p<0.05. Results: Our study included 541 allogeneic HSCT donors who received Neupogen (n=345, 64%) or Nivestym (n=196, 36%) for PBSC mobilization. The median age was 47 (range 17-76) years. The median donor weight was 86 (95% CI 87-91) kilograms. Donors receiving Neupogen had similar pre-GCSF WBC count (median 45 vs 47 K/uL, p=0.739), pre-GCSF CD34 percentages (median 17% vs 17%, p=0.527), and pre-GCSF circulating CD34 count (median 74 vs 74 million cells/kg, p=0.842) compared to donors receiving Nivestym. Neupogen recipients had similar median PBSC product total neutrophil count (TNC, 6.8 vs 6.5 K/uL, p=0.347), product CD34 percentage (75% vs 79%, p=0.068), absolute CD34 count (503 vs 533 million cells, p=0.636), and final CD34 dose (6.1 vs 6.3 million cells/kg, p=0.365) compared to Nivestym recipients. Seven percent (n=28) of donors needed two days of PBSC collection to achieve the target PBSC dose, with a statistically similar proportion noted in Neupogen (n=25, 7.3%) and Nivestym (n=13, 6.6%) cohorts. In a subgroup analysis stratified by age, donors aged 35 or older (n=154) had similar responses in all mentioned variables (p>0.05); however, for donors under the age of 35 years, the CD34 dose was higher in donors receiving Neupogen compared to Nivestym (median 6.9 vs 6.3 million cells/kg, p=0.044). Most of the PBSC collections (98%) were completed in one day, except for five donors in both Neupogen (5%) and Nivestym (8.5%) cohorts. Conclusions: Biosimilar G-CSF (Nivestym) demonstrated similar efficacy for peripheral blood stem cell mobilization compared to the originator G-CSF (Neupogen) among allogeneic HSCT donors. In donors aged 35 years or younger, a slightly lower PBSC product CD34 count was noted with Nivestym compared to Neupogen.
Disclosures
Mahmoudjafari:Omeros: Speakers Bureau; Pfizer, Genentech, Inc., BMS, KITE, Sanofi, Janssen: Honoraria; Genentech, Inc.: Consultancy. Ahmed:Kite/Gilead: Consultancy, Research Funding; BMS: Other: Ad Board. McGuirk:Gamida Cell: Research Funding; Bellicum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; Fresenius Biotech: Research Funding; Novartis: Research Funding; EcoR1 Capital: Consultancy; Magenta Therapeutics: Consultancy; Allovir: Consultancy, Research Funding; Juno Therapeutics: Consultancy; Kite: Consultancy, Research Funding; Pluristem Therapeutics: Research Funding.